For about a million Americans each year, a joint replacement brings relief from pain and restored mobility. But, 5–10% of those people have to endure another surgery within seven years, and most of those are due to an infection in their new joint. If doctors could treat infections more effectively, patients could avoid a second surgery, more pain, and another rehabilitation.
Research conducted by a Boston-based research team has designed and developed a polymer that releases antibiotic. The polymer could be incorporated into the implant itself to sustain and maximize delivery of antibiotic directly to the area of joint replacement.
The new development by Orhun Muratoglu, at Massachusetts General Hospital, and colleagues was reported June 13 in Nature Biomedical Engineering.
"Currently, most infections involving total joint replacement prostheses require a two-stage surgery, in which the patient's daily activities are largely compromised for four to six months," Muratoglu said in a press release. "Our finding that polyethylene, the most commonly used weight-bearing surface in total joint surgery, can be made to safely and effectively release antibiotics implies that fully weight-bearing implants made with this material could be used to treat infection in a single procedure, reducing both the inconvenience and the risk of complications for patients."
Joint Replacement
Joint pain and disability lead patients to consider joint replacement surgery for relief. Joint pain caused by damage to the cartilage lining the ends of the bones most commonly occurs from arthritis or a fracture. Almost 70% of hip replacement operations are done to relieve symptoms of osteoarthritis.
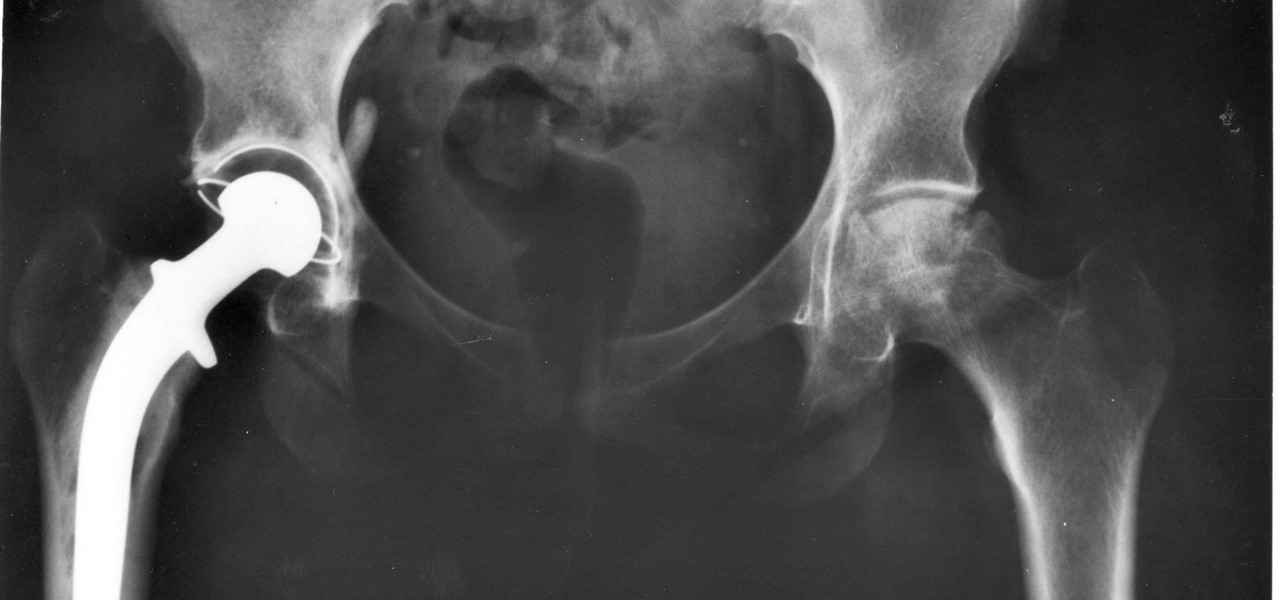
In a total joint replacement procedure, parts of an arthritic or damaged joint are removed and replaced with a metal, plastic, or ceramic device called a prosthesis. The prosthesis is designed to provide the movement and mobility of a normal, healthy joint.
More than seven million people in the US have had a joint replacement procedure. The American Joint Replacement Registry tracks and monitors joint arthroplasty outcomes in 924 participating institutions. Their annual report provides patients and providers with information on the outcomes of joint replacement procedures. According to the report, in 2015 more hip (57%) and knee replacements (61%) were done in women than men. Patients reported their pain and stiffness decreased by half after the surgery.
But, we also know that infections and other mechanical issues, like dislocation or fracture, mean some patients have a 'revision.' For hip replacement patients, that number is about ten percent. Revisions are a kind of a 'redo.' Currently, revision surgery involves removal of the implant and nearby infected tissues. Then, doctors implant a temporary spacer made from antibiotic-releasing bone cement at the bone-implant connection. That implant stays in the joint space for six weeks to six months. After that, the patient gets a new prosthetic joint — which hopefully doesn't become infected.
Bone cement that releases antibiotics has some disadvantages. It doesn't hold up well for long-term weight-bearing, and it doesn't release antibiotic effectively for much more than a week. Attempts at increasing the antibiotic content of the cement weakened its durability.
With such a significant impact on the outcome of joint replacements, scientists have been looking for better ways to prevent or treat infections. The new polymeric material has real potential as a sustained antibiotic delivery system in prosthetic joints.
Treating Infections Before They Happen
The fact that there is no blood flow into the material of artificial joints and no immune cells residing there to fight an infection hampers the treatment of infections in joints. Consequently, when an artificial joint becomes infected, surgery is pretty much the only treatment option.
In an effort to prevent infections from occurring in the first place, a collaborative team composed of scientists, engineers, and surgeons from Massachusetts Institute of Technology, Massachusetts General Hospital, and Harvard Medical School developed an ultra-high molecular weight polyethylene that maximizes drug release while retaining mechanical strength.
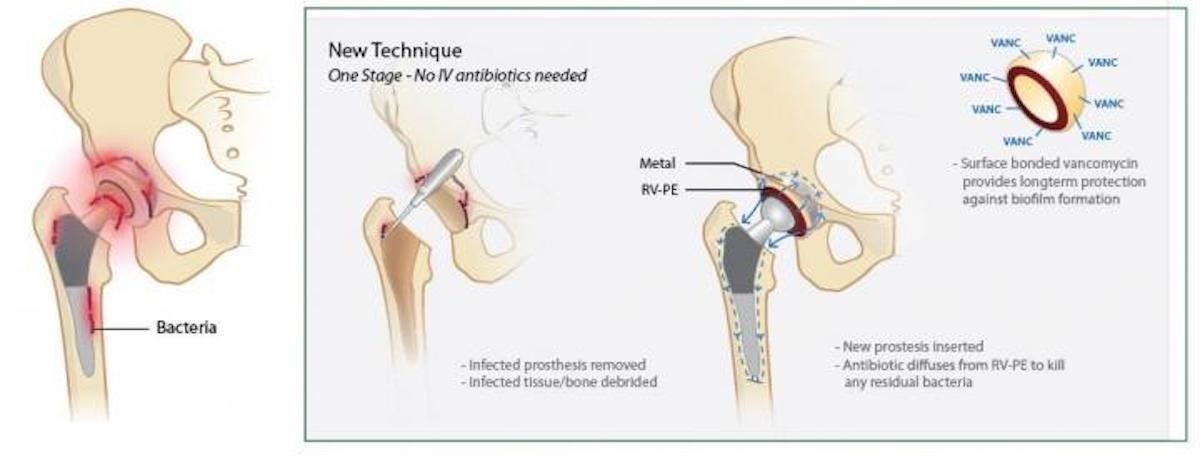
The researchers tested nine different antimicrobials — ciprofloxacin hydrochloride, tobramycin hydrochloride, tetracycline, gentamicin, vancomycin hydrochloride, rifampin, teicoplanin, ceftriaxone, and fusidic acid — by creating clusters of the drugs in the pores of the polymer. They were looking for those with chemical properties that allowed the drug to elute out of the polymeric material in a sustained way, instead of a major hit when first implanted.
It took 30% less vancomycin incorporated into the polymer to create a similar elution rate and antibacterial effect than vancomycin-carrying bone cement, indicating the polymer was more efficient at drug-delivery than bone cement.
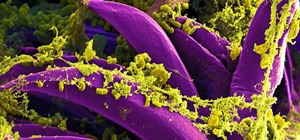
Implants made from this polymer were tested in rabbit models of prosthetic joint infection. Two different types of joint infection models were used. Injection of a solution containing Staphylococcocus aureus into the prosthetic joint mimicked an infection around the implantation site and implantation of a titanium rod covered with a biofilm — bacteria adhered to a surface — of the bacteria represented a particularly difficult to treat infection. If the joint was made with antibiotic-releasing polymer, it successfully cured the infection in both cases. Implantation of an antibiotic-releasing bone cement spacer was not effective.
The study authors said their product is a good candidate for development into a product for use in humans. In clinical trials, its long-term safety and effectiveness will be evaluated before it can be approved by the FDA for use in humans.
Just updated your iPhone? You'll find new emoji, enhanced security, podcast transcripts, Apple Cash virtual numbers, and other useful features. There are even new additions hidden within Safari. Find out what's new and changed on your iPhone with the iOS 17.4 update.




















Be the First to Comment
Share Your Thoughts